
By BD, ISOPP 2022 Virtual Symposium Sponsor
During ISOPP’s 2022 Virtual Symposium in March, BD sponsored a program entitled “Introducing a novel CSTD to protect healthcare workers and reduce contamination across preparation and administration areas in a multi-site health-system.” The purpose of this symposium was to describe the process by which a large, multi-site health-system selected and implemented a new closed system drug-transfer device (CSTD) across their facilities in both preparation and administration areas. The speaker was Erich Brechtelsbauer, PharmD, MS, Assistant Director of Pharmacy at Emory Healthcare and the Winship Cancer Institute in the US.
Dr. Brechtelsbauer began by sharing the ISOPP and US National Institute of Occupational Safety and Health (NIOSH) definition of a CSTD. According to NIOSH, a CSTD is a drug transfer device which mechanically prohibits the transfer of environmental contaminants into the system, and the escape of hazardous drug (HD) or vapor concentration outside of the system.1 The 2007 ISOPP Standards of Practice expanded on the NIOSH definition and state that a closed system is leakproof and airtight and advised that a system cannot be “semi-closed.”2 He elaborated on the design concepts for the CSTDs including a description of the physical barrier type technology as well as the air-cleaning, or vented, technology.3 When evaluating the efficacy of a CSTD, Dr. Brechtelsbauer described his approach was to follow the ISOPP guidance and of “leakproof and airtight” and suggested that a draft NIOSH CSTD vapor containment protocol using isopropyl alcohol4 allows for direct testing of CSTD devices in a standardized way.
Since CSTDs are not yet globally mandated for use when compounding or administering HDs, Dr. Brechtelsbauer gave an overview of relevant global guidelines, standards and regulations that refer to their use. He described USP <800>5 as the only standard that requires use of CSTDs in the US, but other organizations include the use of CSTDs in their guideline and best practice documents, including American Society of Health System Pharmacists (ASHP)6 and the Oncology Nursing Society (ONS)7. These guidelines have similarity in their recommendations because they are based on the hierarchy of controls as a means to reduce exposure. According to the hierarchy of controls, a CSTD is an engineering control, designed to isolate the person from the hazard.2,8 Outside of the US, Dr. Brechtelsbauer described how the ISOPP standards of practice recommend the use of CSTDs and reported on a consensus paper from the Italian Society of Hospital Pharmacy (SIFO) and the Italian Association of Cancer Nurses (AIIAO) that questions the efficacy of air-filtration technology CSTDs and reiterates the requirements in Italian law that requires the use of “closed systems” for preparation and administration of hazardous drugs.9
After discussing the technology and the relevant guidelines and standards, Dr. Brechtelsbauer described the approach he took to selecting and implementing the optimal CSTD for his 11-hospital multi-site health system. Dr. Brechtelsbauer is responsible for the pharmacy areas serving five oncology infusion centers with 170 infusion chairs scheduled with over 400 chemotherapy appointments daily, and approximately 50,000 infusions per year. With such a large facility, Dr. Brechtelsbauer created a CSTD selection committee including key stakeholders (pharmacy, nursing, supply chain, hospital administration) to consider organizational needs, develop a request for information from vendors, and to participate in shared decision making to improve end-user buy in for the product selected. His approach was to allow the nursing and pharmacy departments to decide the CSTD selection based on device effectiveness and ease of use and then move to pricing and contracting discussions after the choices were narrowed based on the clinical decision.
Dr. Brechtelsbauer shared a list of key considerations used when evaluating CSTDs. Included among them were: evidence of effectiveness, drug compatibility, technology and supply integration, ergonomics, HD surface contamination monitoring, as well as implementation and ongoing support. For evidence of effectiveness, the expectation his facility had was that a device would meet the airtight and leakproof CSTD definition of ISOPP, have evidence of microbial ingress, and cleared under the FDA ONB code. In addition to chemical compatibility of the materials of construction of the device with common drug solvents such as DMA, drug compatibility factors also included an evaluation of the volume of drug trapped inside of the CSTD devices as well as ability to use the system with challenging dosage forms such as home infusions or injections. Dr. Brechtelsbauer encouraged the audience to think about future technology changes from the vendors and their systems and how that might impact the institution financially and clinically. He suggested that the number of pieces required for each system could impact the cost of the system for the organization, and so it is an important consideration. For the ergonomic evaluation, he suggested that consideration be given to the force required to apply a vial adapter to the vial, force required to withdraw drug from the vial, force required to inject into an IV container, and finally if the connection mechanism of the system could increase the risk of repetitive motion injury. In his evaluation, they preferred a connection mechanism with a straight push-pull motion as a means to reduce the risk of repetitive motion injury. Additional consideration was given to vendors who could support HD surface contamination monitoring, had demonstrated supply chain reliability, and could support training and implementation across their facilities. To estimate the financial impact of implementation of a CSTD, he used a financial model that calculated the cost per dose for preparation and administration of liquid drugs and the cost per dose for drugs requiring reconstitution using each of the devices being evaluated.
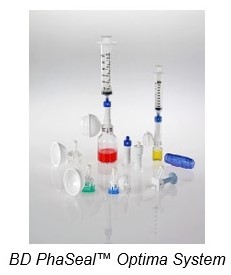
After describing the process, Dr. Brechtelsbauer stated that their hospital selected the BD PhaSeal™ Optima System for their CSTD. Key factors in their decision to select BD PhaSeal™ Optima were: the “push-pull” connection technology to help prevent repetitive motion injury, the vial adapter designed to prevent leaks while allowing more complete vial content withdrawal, compatibility with their infusion and syringe pumps and the convenience of supply management due to few parts required for the system. Being a newer system to the market, his facility was able to gain confidence in the device because they had seen the demonstrated efficacy of the original BD PhaSeal™ System and they felt that the BD PhaSeal™ Optima System was “built upon the demonstrated effectiveness of that original system.”
After describing the selection and implementation of a CSTD, Dr. Brechtelsbauer pivoted his presentation to the ongoing detection and monitoring of HD surface contamination. He described how there is no formal requirement for monitoring HD surface contamination, but then shared the recommendations for HD surface contamination testing given by the 2020 Safe to Touch Consensus conference.10 Key recommendations from the 2020 Safe to Touch Consensus Conference highlighted by Dr. Brechtelsbauer included repetitive testing in the same locations over time to allow trending, employing both qualitative and quantitative tests, sampling for the top 5 to 10 drugs based on facility usage, and standardized reporting of the results. His facility identified 8 vendors who could provide quantitative HD surface contamination testing using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Because the quantitative testing results can take weeks to be reported back to the facility, he described how this technology limits their ability to act on positive test samples.
 Qualitative testing, on the other hand, was described by Dr. Brectelsbauer as a rapid, near-real time test which provides binary results (positive/negative) based on a defined threshold. According to Dr. Brechtelsbauer, the benefit of the qualitative testing is that if a positive contamination is detected, personnel can implement prompt follow up and action planning to decontaminate the affected area and provide feedback to staff as necessary. Currently, there is only one commercially available qualitative HD surface contamination testing system, BD® HD Check, which can test for three hazardous drugs: cyclophosphamide, doxorubicin, and methotrexate. The BD® HD Check system uses lateral flow immunoassay (LFIA) technology, similar to technology used for rapid influenza, Covid-19, or pregnancy tests. A limitation of the rapid testing is that there is no insight into the severity or amount of contamination, so Dr. Brechtelsbauer suggested that a rapid test positive result should be taken seriously and acted upon.
Qualitative testing, on the other hand, was described by Dr. Brectelsbauer as a rapid, near-real time test which provides binary results (positive/negative) based on a defined threshold. According to Dr. Brechtelsbauer, the benefit of the qualitative testing is that if a positive contamination is detected, personnel can implement prompt follow up and action planning to decontaminate the affected area and provide feedback to staff as necessary. Currently, there is only one commercially available qualitative HD surface contamination testing system, BD® HD Check, which can test for three hazardous drugs: cyclophosphamide, doxorubicin, and methotrexate. The BD® HD Check system uses lateral flow immunoassay (LFIA) technology, similar to technology used for rapid influenza, Covid-19, or pregnancy tests. A limitation of the rapid testing is that there is no insight into the severity or amount of contamination, so Dr. Brechtelsbauer suggested that a rapid test positive result should be taken seriously and acted upon.
After implementation of the BD PhaSeal™ Optima System, Dr. Brechtelsbauer conducted an in-use clinical assessment of the novel CSTD in conjunction with a hazardous drug surface contamination analysis. They took 29 wipe samples at two chemotherapy infusion centers within his health system pre- and one-year post BD PhaSeal™ Optima System implementation. The wipe samples were analyzed for doxorubicin, methotrexate, cyclophosphamide, fluorouracil, paclitaxel, and docetaxel using LC-MS/MS to provide quantitative results in ng/cm2. Location A was an infusion center with 70 infusion chairs, and approximately 150 daily infusion appointments, while location B had 15 infusion chairs and 30 daily infusion appointments.
There were 324 sites tested pre- and post-CSTD implementation with a reduction from 50 to 33 sites showing any level of contamination after a year of CSTD usage. Analysis of variance was utilized to compare across time points, and the use of BD PhaSeal™ Optima was associated with a 46% decrease in average LC-MS/MS reported HD surface contamination levels when assessed across sites and HDs (p= <0.0001 ANOVA). In location A, four of five HDs tested showed a reduction in the number of contaminated surfaces after implementation of the CSTD,and all showed reduced contamination levels. At location B, two of five HDs showed a reduction in the number of contaminated surfaces post implementation, while two others showed no contamination pre- and post-implementation.
Overall, Dr. Brechtelsbauer concluded that the BD PhaSeal™ Optima CSTD was associated with a statistically significant reduction in contamination, as well as prolonged contamination prevention. Dr. Brechtelsbauer ended the presentation by recommending that CSTDs be part of an organization’s overarching safe handling initiatives, which may also include rapid HD surface contamination testing to reduce potential exposure.
The complete presentation with question and answer session is available to both ISOPP members and non-members for free on the ISOPP website at: https://www.isopp.org/virtual-library/isopp-2022-virtual-symposium-bd-session-introducing-novel-cstd-protect-healthcare. More information about BD, BD PhaSeal™ Optima System, and BD® HD Check can be found at www.bd.com.
References
- National Institute for Occupational Safety and Health (NIOSH). NIOSH Alert 2004: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf. Accessed January 24, 2022.
- International Society of Oncology Pharmacy Practitioners. Standards of practices: safe handling of cytotoxics. J Oncol Pharm Pract. 2007;13:1-81.
- National Institute for Occupational Safety and Health (NIOSH). Hazardous Drug Exposures in Healthcare: Closed System Drug-Transfer Device (CSTD) Research. https://www.cdc.gov/niosh/topics/hazdrug/CSTD.html. Accessed January 30, 2022.
- National Institute for Occupational Safety and Health (NIOSH). A vapor containment performance protocol for closed system transfer devices used during pharmacy compounding and administration of hazardous drugs. https://www.cdc.gov/niosh/docket/review/docket288/pdfs/a-vapor-containment-performance-protocol-for-closed-system-transfer-devices.pdf. Accessed January 31, 2022.
- United States Pharmacopeial Convention (USP). USP general chapter <800>. Hazardous drugs – handling in healthcare settings. Rockville, MD: United States Pharmacopeia Convention. DocID: GUID-5D76173F-5CB6-47B8-815E-7C275A916085_7_en-US.
- Power LA, Coyne JW. ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm. 2018;75:1996-2031.
- Polovich M, Olsen, M eds. Safe Handling of Hazardous Drugs. 3rd ed. Pittsburgh, PA: Oncology Nursing Society; 2018.
- National Institute for Occupational Safety and Health (NIOSH). Hierarchy of Controls. https://www.cdc.gov/niosh/topics/hierarchy/default.html. Accessed January 31, 2022.
- Società Italiana di Farmacia Ospedaliera e dei Servizi Farmaceutici delle Aziende Sanitarie (SIFO). Management of the risk of exposure of healthcare staff in the handling of injectable antineoplastic drugs: prevention aspects and characterization of safety measures. Italian Consensus Paper. Florence, Italy: Scientific Press; April 2017.
- Gabay M, Johnson P, Fanikos J, et al. Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. Am J Health Syst Pharm. 2021 Aug 30;78(17):1568-1575. doi: 10.1093/ajhp/zxab134.
