
By Emma Foreman, ISOPP Biosimilars Task Force Chair & Winnie Mwangi, Oncology Pharmacist, Kenyatta University Teaching Referral and Research Hospital
In 2019, an ISOPP survey identified several barriers to biosimilar implementation faced by oncology pharmacists internationally. The African region was identified as facing several challenges to implementation that were different from those experienced in higher income settings. This was also raised by participants at the ISOPP Biosimilars workshop which took place at the conference that year. With the help of a focus group of African oncology pharmacists, we designed a survey to explore these challenges further, with a view to designing educational materials and resources to meet the specific needs of oncology professionals in Africa.
Here are the main results: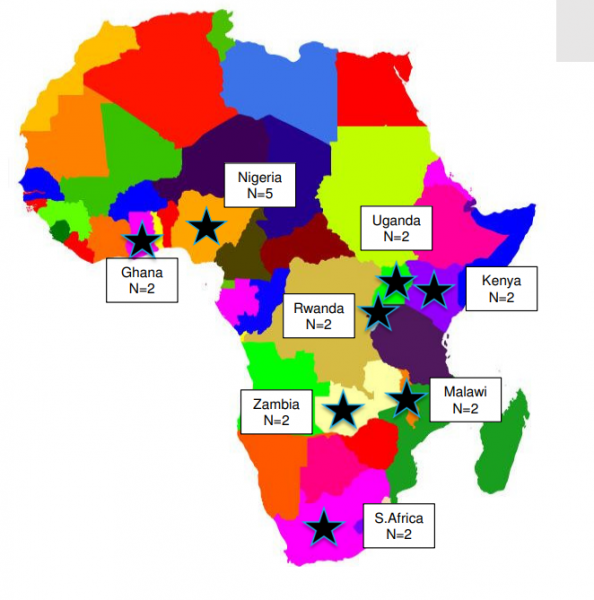
Sixteen responses were received from a range of African countries: Nigeria (n=5), Kenya (n=3), Ghana (n=2), Malawi (n=2), Rwanda (n=1), South Africa (n=1), Uganda (n=1) and Zambia (n=1). The majority (94%) of respondents were hospital pharmacists from a range of institutions including private, government, academic and specialist hospitals.
94% of respondents were already using biosimilars, and all planned to use them in the future. The biggest factor influencing the decision to use biosimilars was cost, with most treatment being funded completely or partly out of pocket or via a health insurance scheme.
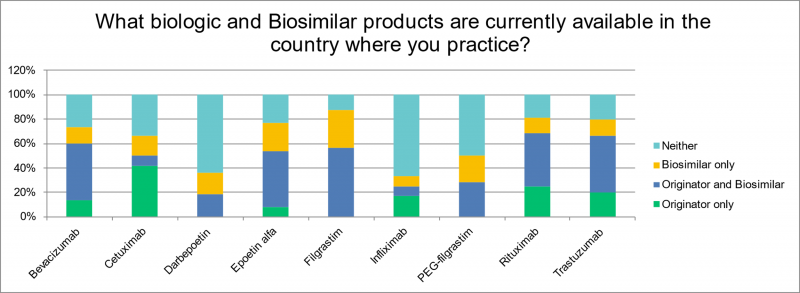
The range of products available varied. Supportive medicine biosimilars such as filgrastim and epoetin were available to 87% and 69% of respondents respectively; around 60% of respondents had access to rituximab, trastuzumab and bevacizumab biosimilars. Infliximab (16%) and cetuximab (25%) were the least available biosimilar products.
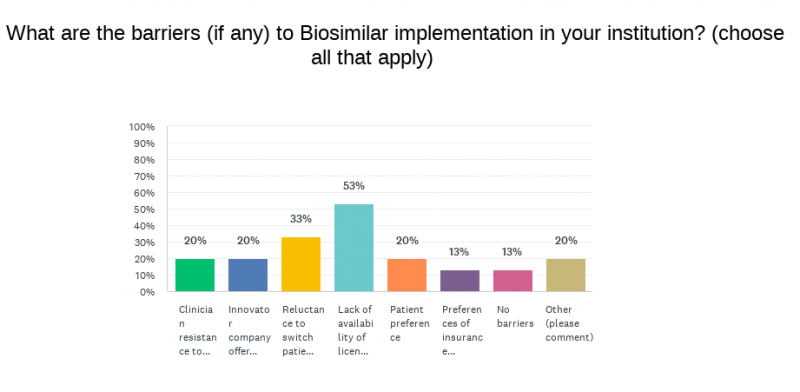
The biggest barriers to implementation were a lack of availability of licensed biosimilar products and the reluctance of prescribers to switch established patients to a biosimilar. A quarter of respondents indicated the availability of unlicensed biologic medicines, known as ‘biomimics.’ Knowledge and awareness of biosimilars was rated as low among both patients and healthcare professionals, highlighting a continued need for education and training.
Suggested resources to address these needs were prescribing guidelines, patient education materials and healthcare professional education materials, with in-person training and webinars being the preferred platform for education.
 In conclusion, our study demonstrates that African pharmacists are keen to use biosimilars in their institutions, seeing cost savings as the main advantage. The main challenges identified were the availability of licensed products, suggesting regulatory issues of problems with the market for biosimilars in Africa, and a lack of understanding about biosimilar products among both patients and healthcare professionals. It would be useful for ISOPP to develop some education and training materials adapted for use in the African region, but further work is required at governmental level to improve biosimilar availability.
In conclusion, our study demonstrates that African pharmacists are keen to use biosimilars in their institutions, seeing cost savings as the main advantage. The main challenges identified were the availability of licensed products, suggesting regulatory issues of problems with the market for biosimilars in Africa, and a lack of understanding about biosimilar products among both patients and healthcare professionals. It would be useful for ISOPP to develop some education and training materials adapted for use in the African region, but further work is required at governmental level to improve biosimilar availability.
