By Hisanaga Nomura, Deputy Manager, Japan Agency for Medical Research and Development
In this report, we published our research regarding occupational exposure to hazardous drugs.1
Since the publication of the ISOPP Standards in 2007, many countries and many facilities have implemented the recommendations for safe handling of hazardous drugs at their pharmacies.2
On the surface, these measures appear to have solved the occupational exposure risk from hazardous drugs; however, the problem still isn’t solved completely at the level of the medical workplace.
For instance, we still need to consider the safe handling of oral anti-cancer drugs for health care workers and caregivers at the patients’ homes. In addition, we should consider the exposure from excretion of bodily fluids like sweat in patients undergoing chemotherapy.
To determine the exposure of patients at home, for this study we conducted an examination of the adhesion of the PPT sheet of the oral anticancer drug lenalidomide taken at home.
Lenalidomide is a widely used cancer drug in the hematology field including multiple myeloma. Most often the patients are prescribed the drug in a pharmacy and actually take it at their home.
We measured the contamination and exposure level of the used lenalidomide sheets across 9 facilities in Japan for this study.
Evaluation of blister package after taking lenalidomide
Two blister packages were collected from each institution following the administration of 5 mg lenalidomide capsules (10 capsules/sheet, no cuts). The blister packages were stored at 2-8°C and transported using a private delivery company. All samples were analyzed at Shionogi Pharma, Inc.
Results
I show the result below the amount of lenalidomide deposited on the blister packages. The median value of lenalidomide detected was 73.2 ng [IQR: 43.7-101.5], and the highest value was 545 ng.
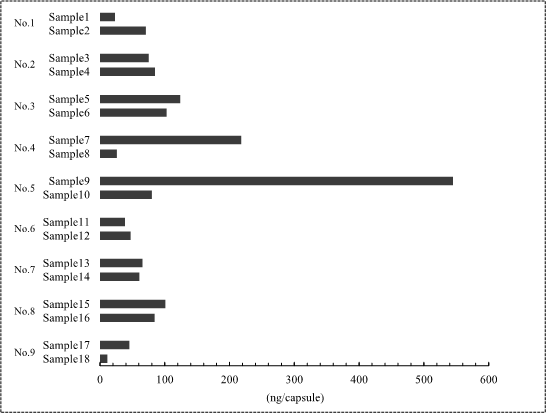
Lenalidomide was detected in all blister packages. Our results suggest that hospital pharmacists are at risk of occupational exposure to lenalidomide.
We had two limitations in this study. The first is that we could not measure the blister package prior to taking lenalidomide. However, we reported an article “Surface contamination of the outer and blister packages of oral anticancer drugs”3 In this study, the contamination on the blister package was not measured. I believe this contamination of lenalidomide occurred after taking lenalidomide in the houses of patients.
The second is that how patients and caregivers handle lenalidomide was not investigated. Therefore, the management status of the blister package at home was unclear.
The result of this study suggests pharmacists should talk to patients and caregivers about how to handle oral anticancer drugs at home, and recommend that they wear gloves during handling starting with retrieval of the blister package until after taking the oral anticancer drugs.
References
- Curr Probl Cancer. 2021 Mar 6:100727.
- J Oncol Pharm Pract. 2007;13 Suppl:1-81.
- J Oncol Pharm Pract. 2020 Jan;26(1):141-145.
