By Hisanaga Nomura, Harbans Dhillon and Bo Yu
Plenary 1: Innovations to transform oncology pharmacy by Harbans Dhillon
Moderator: Kamonsak Reungjarearnrung
This is the abstract of her talk.
There is a need from all over the world to engage and apply new technologies and innovations with limited resources and this calls for a deep involvement and input from oncology pharmacists.
Oncology pharmacy practice is evolving as world situations change over time while facing a pandemic. Social distancing and quarantine measures has necessitated the use of digital health technology to fill and compound prescriptions, track medication data, promote medication adherence, track and manage adverse side effects and to communicate with our patients effectively with real-time patient interaction. However, many patients in Asia do not have access to the internet or have smart devices for this to happen effectively. Thus, innovative methods have to be devised to overcome these challenges.
Oncology pharmacists also have to consider the safety and well-being of our staff when handling and compounding hazardous drugs. Technology is needed to assist in compounding hazardous drugs and automating repetitive tasks in the form of robotics and closed system transfer devices. Again, due to high initial and ongoing costs, it is hard to purchase these expensive equipment. Innovations are needed instead to get around to make the processes more efficient, cost-effective and safer.
As we move away from intravenous oncology drugs to oral chemotherapy, safety again plays a pivotal role. Oncology pharmacists need to remotely monitor the use of oral chemotherapy as well as concomitant drug therapy, adverse side effects and promote medication adherence. Patients have to be taught safe handling guidelines and safe disposal techniques when taking oral medication to their homes. Digital health plays an important role in optimizing treatment by sending medication reminders, promote patient monitoring and telemedicine services. This will help reduce costs through wastage and non-compliance, improve adherence, manage side effects better and overall improve health.
Plenary 11:
Innovations and Practices
Innovation Application of Technology in Oncology Pharmacy
Moderator: Harbans Dhillon
This Plenary was to showcase the main innovations in compounding of hazardous drugs and was well presented by Hisanaga Nomura from Japan and Bo Yu from China.

Speaker 1: Hisanaga Nomura (National Cancer Center Hospital East, Japan) - Handling Hazardous Drugs with Closed System Transfer Devices
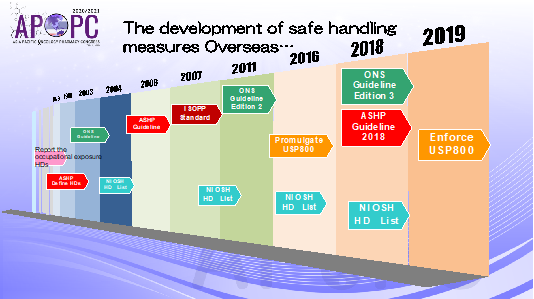
Number of people who handle anticancer drugs in their profession is increasing. Anticancer drugs, which are hazardous drugs (HD), exert cytocidal effects on cancer cells, but many have also been shown to have mutagenicity, teratogenicity and carcinogenicity; therefore, safe handling of anticancer drugs is necessary. Occupational exposure to hazardous drugs and the potential health risk to healthcare workers first became a recognized safety concern in the 1970s. Overseas, a multitude of national organizations, both governmental and professional, continue to release and update guidelines for the safe handling of hazardous drugs. Various societies have published guidelines including the ASHP, ONS, and ISOPP. Most important, however, is USP 800 from the US. (Slide 1)
USP 800 is the standard governing HDs and their handling during receipt, storage, compounding, dispensing, administration, and disposal of both sterile and nonsterile preparation. It is in place to protect personnel and environments from HDs compounds, such as those used in chemotherapy drugs or radiopharmaceuticals. Furthermore, it focuses on the safe handling of HDs to minimize the risk of exposure to healthcare personnel, patients, and the environment.
USP 800 describes CSTDs must be used for administration of antineoplastic HDs when the dosage form allows. Techniques and ancillary devices that minimize the risk posed by open systems must be used when administering HDs through certain routes.
Slide 1:
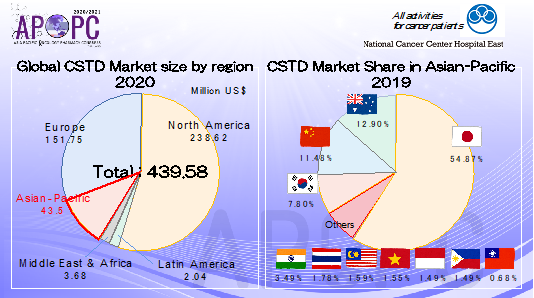
According to one external research organization, the majority of the global CSTD market sales come from North America and the EU. (Slide 2) These two figures show graphically how the utilization of CSTDs has only really begun to spread out into the Asian-Pacific region. CSTD market share in Asian-Pacific is almost Japan. Other Asian countries is not enough spread of utilization.
General occupational health and safety practices do not suggest safe levels of cytotoxic exposure in current evidence. CSTDs must be used to limit exposure until there is sufficient evidence to prove that other practices can ensure the safety of the operator. I am convinced that disseminating CSTD to pharmacies will lead to the construction of a safe environment.
Education on the safe handling of HDs is essential to ensure adequate safety measures in each country. To achieve this education goal, it is necessary for each country to have its own guidelines regarding the safe handling of CSTDs. The spread of the use of CSTDs in the pharmacy department will help lead pharmacists to avoid exposure.
Slide 2:
Speaker 2: Bo Yu (Shanghai Tongren Hospital) - Presentation Title: Pharmacy Automation
Pharmacy automation involves the mechanization of processes, of which can include medication dispensing, packaging, labeling, storage, and retrieval of medications. When dealing with pharmacy automations, we must optimize human labor, which is the key point in large hospitals. When new technologies are introduced, we always expect better management of supply chain, faster and more safe process, and of course much improved experiences with nurses. However, what we suffer most is that after spending a huge amount of money, we get machines and machines, which will not be added up to a robot or robots to do some team works. The only result we get, most of the time, is machines could do part of a human’s job and need someone to look after it. However, in the realistic world like here in CHINA, we are expecting some REAL robot to bring the solution-ship for hundreds of Chemo per day, thousands of inpatients infusion per day, and hundreds of trials’ compounding per day. Considering these, none of the choices from current market is feasible. We managed to design and build a prototype to integrate dispensing, including oral boxed medicine and vials, transit, and robotic compounding together. In the talk, two video clips were showed. In summary, I thought there were not true breakthroughs in the past decade. A machine/robot link all the pharmacy supply chains and integrate all movements is expected.

