By Felice Musicco, Instituti Fisioterapici Ospitalieri, Italy
In general, the use of drugs is desirable, taking into account risks and benefits, always within controlled trials, in order to produce valid results and, above all, to produce therapeutic recommendations.
In addition, programs for expanded access to experimental drugs or compassionate protocols should be proposed by central agencies after careful evaluation of evidence of risks and benefits, so as to involve manufacturers and facilitate procedures for access and supply to citizens through pharmacies and pharmacists.

 Image from https://www.wxyz.com/
Image from https://www.wxyz.com/
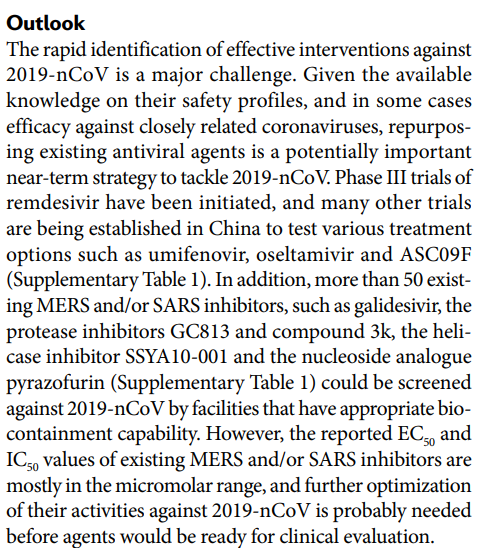
- A good article has been recently published in Nature Review Drug Discovery (link). The similarity with respect to SARS and MERS (severe acute respiratory syndrome coronavirus SARS-CoV and Middle East respiratory syndrome coronavirus MERS-CoV) suggest to propose some drugs tested for these infections. As shown in this table attached to the article, no drugs are already approved for these indications, however many drugs are in the preclinical phase or phase II, III or IV trial. Some are direct antiviral agents, others instead act by improving the antiviral immunological defenses. In some cases combinations with drugs with both mechanisms of action are being tested. The lopinavir and ritonavir combination is available in Italy, indicated in combination with other antiretroviral medicines, for the treatment of adults, adolescents and children from 14 days of age with human immunodeficiency virus (HIV-1) infection. Some interferons and chloroquine are also approved for other pathologies. The article concludes:
- Another interesting article is available at this link. The conclusions:

- The Chinese guidelines issued in January, in the section on drug treatment report:
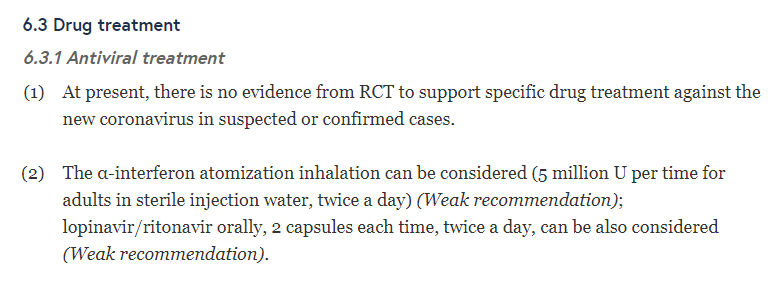
Weak recommendation means:

So there is uncertainty about the advantages or disadvantages of using these drugs.
- The NICE webpage - UK National Institute for Health and Care Excellence, updated on March 10th states:

- In Italy the Istituto Superiore di Sanità which, on this page (update February 2) reports similar statements:

"Treatments
There are no specific treatments for coronavirus infections and there are currently no vaccines available to protect against the virus. Most people infected with common coronaviruses heal spontaneously. With regard to the new coronavirus COVID-19 there are no specific therapies; the symptoms of the disease (so called supportive therapy) are treated in order to promote healing, for example by providing respiratory support."
- In the United States, the National Center for Immunization and Respiratory Diseases (NCIRD) reports recommended treatments here (update from March 7)

The section on experimental treatments reports the following:

- Very interesting guidelines updated on January 28 can be downloaded from the WHO website at this link. In the treatments section you will find:
8. Specific anti-Novel-CoV treatments and clinical research
There is no current evidence from RCTs to recommend any specific anti-nCoV treatment for patients with suspected or confirmed 2019-nCoV infection.
Unlicensed treatments should be administered only in the context of ethically-approved clinical trials or the Monitored Emergency Use of Unregistered Interventions Framework (MEURI), with strict monitoring. https://www.who.int/ethics/publications/infectious-disease-outbreaks/en/
Clinical characterization protocols are available, at the WHO 2019 nCoV website: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. WHO has established Global 2019-nCoV Clinical Data Platform, for member countries to contribute. Contact EDCARN@who.int for additional questions.
- It is interesting to read this EMA document link "EMA plan for emerging health threats". In particular see:

- Other interesting articles were published in late February on Medscape, available at this link, on Nature at this link, and on MVJ at this link
