By Lorraine Diwas and Margarita Padilla, Inpatient Pharmacy, Cleveland Clinic Abu Dhabi, United Arab Emirates
Introduction
The Cleveland Clinic Abu Dhabi (CCAD) is a multi-specialty hospital located in Abu Dhabi, United Arab Emirates that has been open to the public since May 2015. CCAD has five Centers of Excellence in the following Institutes: Heart & Vascular, Neurological, Digestive Disease, Eye, and Respiratory & Critical Care. Other Institutes include Medical Subspecialties (including Oncology and Hematology), Surgical Subspecialties, Emergency Medicine, Pathology & Laboratory Medicine, Imaging, Anesthesiology, and Quality & Patient Safety. Cleveland Clinic Abu Dhabi is the first UAE healthcare facility, and the second in the GCC, to achieve HIMSS Stage 7. HIMSS Stage 7 is an international benchmark for the use of advanced IT in order to improve patient care. There are eight HIMSS stages (0 – 7), with the final Stage 7 representing the most advanced patient record environment.

The department of pharmacy services in CCAD is committed to provide high quality, evidence-based and patient-centered pharmaceutical care to our patients by assuring the use of safe, effective and cost-effective medication therapy according to the local and international guidelines. In this brief report, we will focus on the main elements of our chemotherapy preparation unit in terms of workflow, training, safety measures and technologies.
Training and Workflow
Before handling chemotherapy medications, the pharmacy caregiver must complete a mandatory training consisting of theoretical and practical parts that covers all the necessary elements of the chemotherapy preparation process including aseptic techniques, handling precautions, PPEs and proper handling of such medications to ensure safety of the caregiver and to maintain the quality of the prepared medications. This training should be documented in the caregiver’s file and updated annually to maintain continuous competency in terms of knowledge and skills in preparing chemotherapy medications.

Below are the major steps according to the current workflow:
Whenever an order is verified the production label will print on the hazardous medication printer in the SPC (production label will have a barcode).
The technician will collect the required medications and fluid to be prepared at that time.
The technician will scan the production label and each ingredient of the chemo to be prepared at that time. He/She should not scan and then set aside.
After finalizing the dispense preparation, the bag labels will be printed on the hazardous printer inside the Hazardous room.
The technician will start preparing the required amounts of each medication and then the pharmacist will double check them in the biological safety cabinet.
The technician will add the checked ingredients to the bag and stick the label on the bag in the presence of the pharmacist.
The pharmacist will scan using the medication tracking system scanners to update the status of the order as prepared.
The pharmacist will double check the final product, scan on dispense check and place it in the pass through window for delivery.
Safety Measures
- Closed System Drug-Transfer Device (CSTD):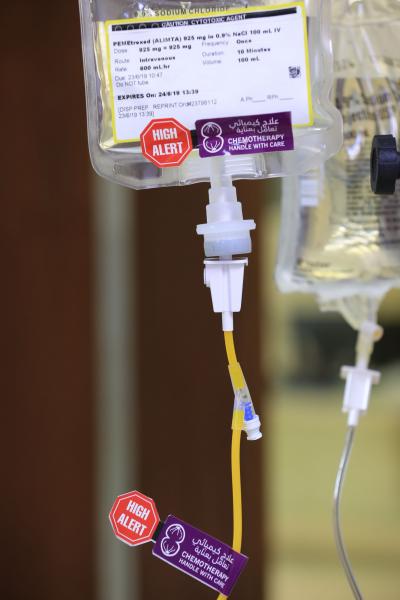
A CSTD is a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of the hazardous drug or vapor concentrations outside the system. These devices include: connectors, adaptors, injectors…etc.
- Chemotherapy specific personal protective equipment:
This includes a second set of chemo gloves on top of the standard gloves, closed front chemo gown, a second pair of shoe covers, a fit-tested NIOSH-certified N95 or more protective respirator and eye protection (goggles) when handling corrosive volatile materials or loose hazardous powders.
- Biological safety cabinets:
A ventilated cabinet for CSPs, personnel, product, and environmental protection having an open front with inward airflow for personnel protection, downward high-efficiency particulate air (HEPA)-filtered laminar airflow for product protection, and HEPA-filtered exhausted air for environmental protection.
- Auxiliary labels and chemotherapy bags:
Packaging and subsequent labeling designed for sterility and stability until BUD of medication and for patient safety.
- Spill kit:
A collection of items to be used in case of a spill. Each item is developed so that a prompt response and clean-up may be performed. Typically a kit would consist of absorbent pads, a scoop or scraper, a solidifying chemical, disinfectant wipes, drying wipes, gloves, shoe covers, face shields, disposable gown and biohazard bags.
- Particle count, temperature, air flow and humidity monitoring:
These elements must be monitored and documented on a routine basis to ensure adequate environmental and personnel controls are in place to prevent contamination of CSPs.

Different types of plastic bags with proper labeling used to dispose waste from chemotherapy preparation.
Technologies
- Computerized provider order entry CPOE:
Our CPOE is equipped with safety alerts and clinical support tools that assure safe ordering and dispensing of chemotherapy including proper dosing, updated drug information links, administration instructions, drug interaction checker, and preparation instructions.
- Automated Dispensing Machines:
Automatic dispensing machines (ADM) or automated drug cabinets (ADC) are a computerized drug storage and dispensing device used in health care settings like hospitals and nursing homes, and are located at the point of care (the acute care wards, ICU, ED, clinics and OR) rather than in the central pharmacy.
- Medication tracking system:
A special system that consists of barcode scanners and software that is used to track the prepared medication from the moment it is verified until it is handed over to the nurse. This system is very important and can help in following the status of each dose, to set expectation about preparation time with nurses and to identify delivery and turn around time.
- Barcode scanning system:
Barcoded Medication Administration (BCMA) is an inventory control system that uses barcodes to prevent human errors in the distribution of prescription medications at hospitals. The information encoded in barcodes allows for the comparison of the medication being administered with what was ordered for the patient.
• The labeling photos are courtesy of Rabih Dabliz, PharmD, FISMP; Senior Manager, Quality & Medication Safety Services
